ROMA :
Even in combination serum biomarkers have limited sensitivities and specificities, so efforts to improve risk stratification tools for women with pelvis masses have incorporated other factors from woman’s clinical evaluation. Moore and colleagues developed an algorithm incorporating CA 125, HE4 and menopausal status, called Risk for Ovarian malignancy Algorithm (ROMA) which has been cleared by FDA as an aid in assessing whether a premenopausal or postmenopausal women who presents with an ovarian adnexal mass is at high or low likelihood of finding malignancy upon surgery.
ROMA uses the results for HE4 and CA125 to generate apredictive index (PI) for OEC calculated by thefollowing formulas:
For premenopausal women:
PI = -12.0 +2.38 *ln(HE4) + 0.0626 *ln(CA125)
For postmenopausal women:
PI = -8.09 +1.04 *ln(HE4) +0.732 *ln(CA125)
Then, ROMA value is calculated as follows:
ROMAvalue (%)= exp(PI)/[1 +exp(PI)] *100
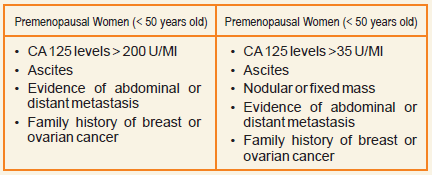
According to the indications of the HE4 manufacturer,indexes of at least 7.4% and 25.3% indicate a highrisk for the presence of EOC in pre- or postmenopause, respectively.
Moore et al. reported that ROMA successfully classified women presenting with a pelvic mass into low and high risk groups with 93.8% of women who were found to have OEC to be correctly identified as high risk. Most recently, Moore and Colleagues compared ROMA to the Risk of Malignancy Index (RMI)that is comprised of the measurement of CA 125 levels in addition to an imaging score and assessment of menopausal status to predict OEC women presenting for surgery with a pelvic mass. At a set specificity of 75% ROMA demonstrated a sensitivity of 89% compared to 80.7% of RMI, a difference which was statistically significant (p=0.0113).
A recent metaanalysis [7] found that, first, ROMA could help distinguish OEC from benign pelvic mass with a high diagnostic accuracy (AUC: 0.93). The ROMA has high sensitivity to predict advanced stage OEC than early stage OEC and in postmenopausal women than in premenopausal women. Second, although HE4 has higher specificity than CA125 for OEC monitoring, CA125 has better diagnosis accuracy (higher AUC) than HE4 for OEC or OC prediction. This is based on the results of 4 studies that compare HE4 and CA125 within the same population. Third, based on the results of comparison of HE4, CA125 and ROMA in the same population, the overall performance (AUC) of the three tests for OEC prediction are similar. ROMA inherits the strengths and weakness of CA 125 and HE4 alone. Indeed, ROMA is more sensitive than HE4 but less sensitive that CA 125 and it is more specific than CA 125 but less specific than HE4.
Key points:
- Ovarian epithelial cancer (OEC) is the deadliest of the gynecological cancers with the majority of patients diagnosed at an advanced stage.
- Patients with OEC show improved outcomes when surgery is performed by an experienced gynecological surgeon or at experienced OEC treatment centers.
- Currently CA 125 is the biomarker that is used to aid in the management of OEC patients but the marker has limited sensitivity and specificity.
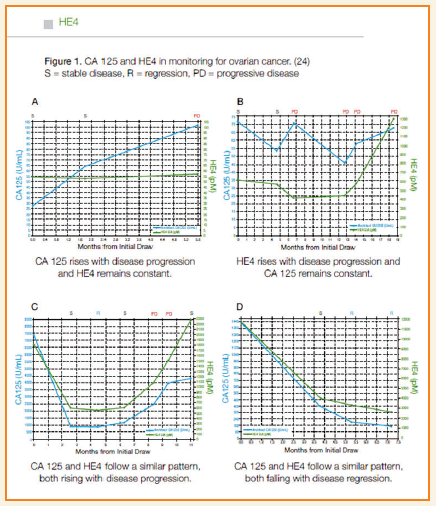
- HE4 is a new biomarker for OEC and has been approved by FDAin monitoring recurrence or progression in OEC patients.
- HE4 is also used with CA 125 in an algorithm called Risk of Ovarian Malignancy Algorithm (ROMA).
- ROMA is an aid in assessing the risk of malignancy upon surgery in womenwho present with an ovarian adnexal mass,patients at high risk can have their surgeryperformed by an experienced gynecological surgeon or at an experiencedOEC treatment center for better outcome.